Abstract
Background: With the advent of immunomodulatory agents (IMiDs), proteasome inhibitors (PIs) and, more recently, anti-CD38 monoclonal antibodies (mAbs), prognosis of patients with multiple myeloma (MM) has improved considerably. Unfortunately, even with these 3 major MM drug classes, most patients ultimately relapse and require further therapy. There remains an incomplete understanding of how patients who have received extensive therapy and with relapsed/refractory multiple myeloma (RRMM) are treated in routine clinical practice, as no standard-of-care exists for these patients, and what the outcomes are in this real-world setting.
Objective: This study aims to evaluate the outcomes of patients with triple-class (IMiD, PI and anti-CD38 mAb) and triple-line exposed RRMM using real-world data from patients in Belgium.
Methods: A multicenter, observational study, involving 7 non-academic and academic Belgian centers, was conducted based on a retrospective chart review of adult RRMM patients who started subsequent treatment from March 2017 through May 2021 after having received ≥3 lines of therapy including at least an IMiD, a PI, and anti-CD38-directed therapy (tri-exposed). Data were captured in an electronic case report form (Castor EDC). Patients with an ECOG performance status of ≥2, who received prior CAR-T treatment or prior BCMA-targeted therapy, or with a known active or prior history of CNS involvement (or with clinical signs thereof), were excluded. All treatment lines initiated after becoming eligible were used in the analysis. Specifically, all treatment lines for patients meeting the eligibility criteria more than once in their entire follow-up were included as separate observations, with date of treatment initiation as specific baseline for each treatment line. Cox proportional hazards models were fitted to explore the prognostic value with Overall Survival (OS), Progression Free Survival (PFS), and Time to Next Therapy (TTNT).
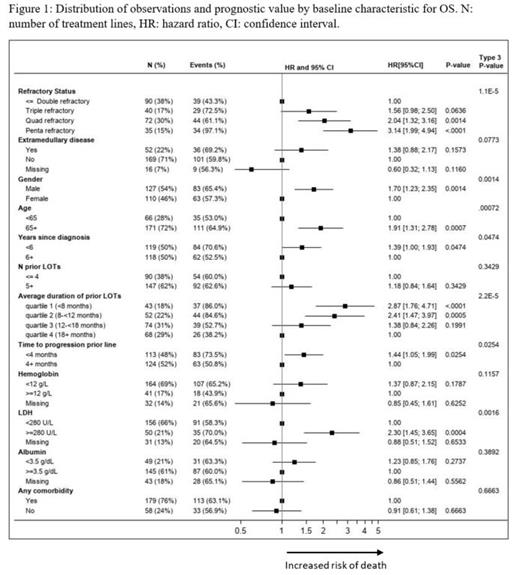
Results: A total of 112 patients with 237 eligible treatment lines were included in the analysis; median follow-up was 16.6 months. In 45% of the initiated treatment lines, patients were refractory to 4 or 5 therapies, 62% had received ≥5 prior lines, 22% had extramedullary disease and in 48% of observations the time to progression in prior line was shorter than 4 months. After patients became tri-exposed, more than 50 unique treatment regimens were initiated, with the following being the most common: carfilzomib + dexamethasone (14%), pomalidomide + dexamethasone + chemotherapy (8%), and ixazomib + lenalidomide + dexamethasone (6%). Additionally, 4% of included observations were exposed to anti-BCMA agents. Overall, the following treatment classes were the most frequently started: PI only (19%), PI + IMiD combinations (17%), and regimens including anti-CD38 antibodies (15%). Median OS was 9.79 months [95% CI: 7.79; 12.22], median PFS was 3.42 months [95% CI: 2.79; 4.27], median TTNT was 3.61 months [95% CI: 3.09; 4.57]. Higher refractory status (p<0.001), being male (p=0.001), older age (p<0.001), shorter duration of prior lines (p<0.001), shorter time to progression in prior line (p=0.025), and higher LDH levels (p<0.002) were prognostic for worse outcomes for both OS (Figure 1) and PFS.
Conclusions: This retrospective chart review of patients with tri-exposed RRMM in Belgium shows that real-world outcomes in terms of OS, PFS and TTNT are poor for these patients, with a median OS of <10 months. A wide variety of treatment regimens used in clinical practice confirm the absence of a clear standard-of-care in this patient population. The literature also confirms that these poor outcomes observed in Belgium, for this subset of MM patients, are similar in other countries. These real-world data highlight the high unmet medical need in this patient population and critical need for new and effective treatment options.
MD and MCV contributed equally to this work.
Delforge: Amgen, Celgene, Janssen, Sanofi: Honoraria, Research Funding. Vekemans: Amgen: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; BMS-Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen Pharmaceutica: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees. Depaus: Takeda: Consultancy; Novartis: Consultancy; Janssen: Consultancy; Celgene: Consultancy. Meuleman: iTeos Therapeutics: Consultancy. Strens: Realidad bvba: Consultancy. Van Hoorenbeeck: Janssen: Current Employment. Moorkens: Janssen-Cilag: Current Employment. Diels: Janssen: Current Employment. Ghilotti: Janssen-Cilag SpA, Cologno Monzese, Italy: Current Employment. Dalhuisen: Janssen: Current Employment. Vandervennet: Janssen: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal